Background: While advances in molecular cytogenetic assessment and incorporation of recurrent chromosomal abnormalities with prognostic significance into risk stratification have improved outcomes for children with acute myeloid leukemia (AML), outcome disparities persist. Specifically, Latino children are noted to have inferior outcomes compared with their non-Latino peers. These disparities may be related in part to ethnic-based differences in cytogenetic features, but the prevalence of AML cytomolecular subtypes within specific demographic populations is a relatively understudied area. Our aim is to evaluate the association between self-reported race/ethnicity and recurrent prognostic cytogenetic features in pediatric AML.
Methods: We analyzed data from the multi-institutional Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium between 2007 to 2022. REDIAL includes children newly diagnosed with AML at six major pediatric cancer centers in the Southwestern U.S. Demographic and clinical characteristics include self-reported race/ethnicity, sex, and age at diagnosis. Cytogenetic subtypes evaluated included KMT2A rearrangements, FLT3 mutation, PML::RARA, RUNX1::RUNX1T1, CBFβ::MYH11, CEBPA, DEK::NUP21, RBM15::MKL1, RPN::1EV11, del5q, monosomy 5, monosomy 7, and BCR::ABL1. Features were dichotomized as present or absent in those that were tested, and subtypes categorized into favorable, unfavorable, and neutral groups. Multivariate logistic regression models were used to estimate adjusted odds ratios (aORs) and 95% confidence intervals (CIs), adjusting for sex and age at diagnosis.
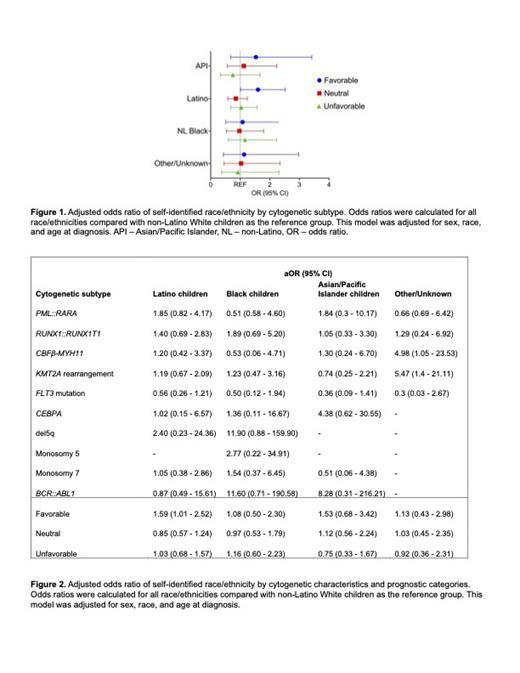
Results: Among the 665 AML patients included in this analysis, 46.3% self-reported as Latino, 32.6% as non-Latino White (NLW), 9.4% as Black, 6.4% as Asian/Pacific Islander (API), and 5.1% as other/unknown. The mean age at diagnosis was 7.9 years (standard deviation 6.3); a majority were male (51.6%). Among all patients that were tested, 20.8% (134/644) had at least one favorable subtype, and 27.3% (161/590) had at least one unfavorable subtype. Adjusted logistic regression models showed Latino children had 1.59 times greater odds of having at least one favorable subtype compared to NLW children (aOR 1.59, 95% CI 1.01-2.51) but were not at increased odds of having at least one unfavorable subtype (aOR 1.03, 95% CI 0.68-1.56) or a neutral subtype (aOR 0.84, 95% CI 0.-57-1.23) compared to patients of other race/ethnicity. While multivariable analyses of specific subtypes did not meet the threshold for statistical significance, there were some notable trends. For instance, compared with NLW children, Latino and Black children had a higher prevalence of the favorable RUNX1::RUNX1T1 translocation (aOR: 1.40, 95% CI: 0.70-2.83; 1.90, 0.69-5.20, respectively). Compared with NLW children, Latino and Black children also had a higher prevalence of the favorable CEBPA mutation (aOR 1.02, 95% CI 0.15-6.56; 1.36, 0.11-16.6, respectively), and were more likely to have unfavorable KMT2A rearrangements (aOR 1.18, 95% CI 0.67-2.08; 1.23, 0.47-3.1, respectively). Additionally, Latino children were more likely to have the favorable translocation CBFβ::MYH11 compared with Black and NLW children (aOR 2.2, 95% CI 0.27-18.1; 1.20, 0.42-3.37, respectively), and less likely to carry the unfavorable FLT3 activating mutation compared with their NLW peers (aOR 0.56, 95% CI 0.26-1.21).
Conclusions: Our findings suggest Latino children are more likely to have a favorable cytogenetic subtype than NLW children, which suggests disparities in outcomes are not primarily due to underlying differences in prognostic subtypes. Additional analyses of pediatric AML as it relates to cytogenetic subtypes and race/ethnicity is ongoing, including the incorporation of disease response and treatment outcomes, to understand how these factors may impact disparities in outcomes.
Disclosures
Huynh:Servier: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal